

My fourth grade teacher was a child of Irish immigrants, and sometimes he told stories in class. My favorite went like this: leprechauns are bound by a law that obliges them to do whatever you say if you catch them. One day, a man caught a leprechaun and ordered him to reveal the location of his pot of gold. The leprechaun was furious, but he had no choice. He led the man through the forest to an old tree and said, “There. Me treasure is under the roots of that tree,” and that was the truth.
The man needed an axe and a shovel to get at the gold, so he tied a ribbon around the tree to mark it while he went back to town for some tools. He commanded the leprechaun not to take down the ribbon or move his gold or anything like that. The leprechaun, still under obligation, grumbled but agreed. When the man returned with his tools, the tree was still marked by its ribbon, but so was every tree in the whole forest. He never found that treasure.
The beauty of the leprechaun’s trick is that a completely ribboned forest has exactly as much information as an unribboned forest. A ribbon on every tree except one would convey as much as a ribbon on one tree. This “amount of information” is called entropy, and it is as much a physical quantity as length, voltage, and temperature. In fact, I think that entropy is a more fundamental concept than temperature— knowing about entropy makes it easier to understand what temperature is.
The most useful physics to know for cooking is the fact that heat
is different from temperature. Heat is a thing, it’s a form of
energy, almost a substance. It flows from the burner to the pot of
boiling water and spreads throughout the room. It has to go
somewhere; it can’t just disappear. Heat can change into other
forms of energy, or even matter— a nuclear power plant turns
matter into heat.

Temperature, on the other hand, is more abstract. It is a quantity that is equalized when things touch: ice and tea exchange heat until they have the same temperature. So does fudge and a candy thermometer, but the candy thermometer assigns arbitrary numbers to this mysterious quantity. Liquids boil and foods burn at specific temperatures, not amounts of heat, so it’s an important number. The dials on most stoves set the rate of heat flow, rather than temperature, so higher settings should be used for something watery (water absorbs a lot of heat per degree of temperature) than for something oily (oil absorbs less heat for the same temperature).
Though we use temperature every day, it has no meaning other than marks on a thermometer unless we first know about entropy. Entropy is sometimes described as disorder or randomness, but that’s not always a useful way to think about it. When I add memory chips to my computer, it is to increase the computer’s entropy, but I don’t want the computer to become random or disorderly!
 The leprechaun’s trick provides a great example of entropy. The
forest’s entropy can be defined as the number of ways to tie ribbons
on trees, within a restriction. For instance, if the
restriction is that the leprechaun can only use one ribbon, then
there are as many ways to do it as there are trees in the forest. A
meaning could be associated with the tree that gets the ribbon, such
as “thar be gold here,” but it is not necessary.
The leprechaun’s trick provides a great example of entropy. The
forest’s entropy can be defined as the number of ways to tie ribbons
on trees, within a restriction. For instance, if the
restriction is that the leprechaun can only use one ribbon, then
there are as many ways to do it as there are trees in the forest. A
meaning could be associated with the tree that gets the ribbon, such
as “thar be gold here,” but it is not necessary.
If the leprechaun is allowed to use two ribbons, then there are many more ways to do it: a thousand-tree forest has almost a million (1000 × 999 = 999,000) ways to hang two ribbons. With enough ribbons, the leprechaun can encode complex messages. Ribboned and unribboned trees could represent ones and zeros in a binary sequence, as in a computer. But if he is forced to use nearly as many ribbons as there are trees, his options become limited again. With 999 ribbons in a thousand-tree forest, there are only a thousand ways to pick the tree that doesn’t get a ribbon. And there’s only one way to tie a ribbon on every tree, so if he must use one thousand ribbons, one per tree, the leprechaun cannot convey any information at all. “To the constant consternation of extortionists and thieves,” he says, tamping his pipe.
Calling entropy an “amount of information” may sound
more applicable to computers than to a pot of water heating on the
stove, but it’s useful for both. A pot of water is made of atoms,
and atoms have locations and velocities— that is, information.
The number of ways to arrange the positions and velocities of all
the atoms is beyond astronomical.  Instead of dealing with big
numbers, we just count digits: one million has six digits, ten
million has seven digits, etc. Entropy is proportional to the
number of digits in the number of combinations.
Instead of dealing with big
numbers, we just count digits: one million has six digits, ten
million has seven digits, etc. Entropy is proportional to the
number of digits in the number of combinations.
Not every combination of atom velocities is relevant for the pot of water, however. Fast-moving atoms have more energy than slow-moving ones, so some combinations would add up to more energy than the pot really has, some less. Only the combinations with the right total energy should be counted. This restriction is like specifying the number of ribbons that the leprechaun must use in his forest. Just like the ribbons, the amount of energy dictates the number of possible combinations. As more energy becomes available, the number of combinations typically grows, faster for some materials than for others. This is the essence of temperature.
Temperature is the amount of heat energy we’d have to add to a material to change its entropy by a given amount. If you are familiar with calculus, it is the derivative of heat with respect to entropy. If not, consider the graph below. Entropy is the horizontal axis, energy is the vertical, and two different materials have relationships between energy and entropy given by the two curves. Temperature is the steepness of the curves.
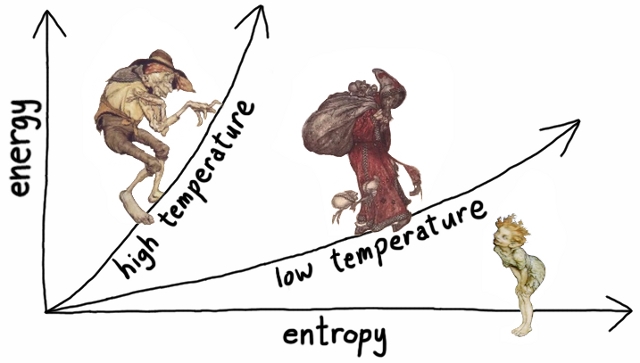
For most substances, having a lot of heat means having a high temperature, but this is not always the case.
The most direct physical analogue of the leprechaun’s forest is a laser beam. In a laser, a substance is restricted to only two energy levels (or only a few). The laser light comes from atoms dropping from the high-energy state to the low-energy state. Think of the atoms in the high-energy state as trees with ribbons on them and those in the low-energy state as trees without ribbons.
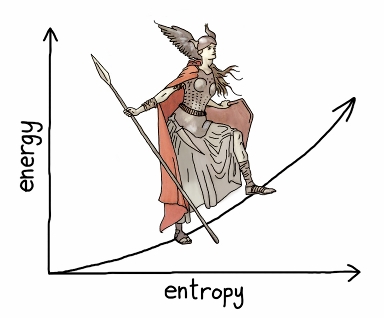
When a laser is off, all or most of the atoms are in the low state. To turn on the laser, we start adding energy to the system. It is now like a forest with a small number of ribbons. Each new ribbon increases the number of possible combinations, so the relationship between energy and entropy looks like this:
As we continue to pour energy into the system, the number of possible atomic combinations grows— up to a point. When the laser reaches half-power, there are as many high-energy atoms as low-energy atoms, all jumping up and down, rapidly exchanging energy. As with the ribbons in the forest, this is the point of maximum entropy. Adding more energy reduces the number of combinations. At full power, nearly all of the atoms are in the high-energy state, so the number of possible combinations is small again, like a forest full of ribbons. The graph looks like this:
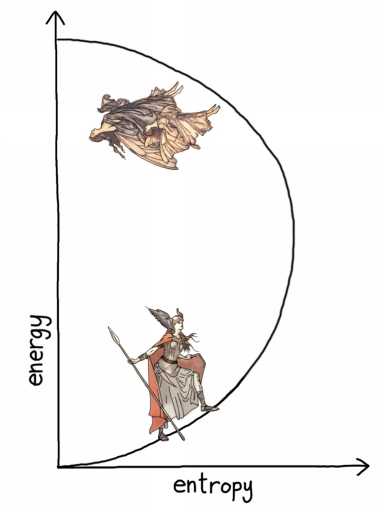

“Blight, Bright, and Spigot activated the laser cannon, whereupon it glowed and hummed faintly.”
Since the curve has doubled back on itself, the temperature is negative— less than absolute zero! The laser in your CD player routinely breaks the supposed law that nothing can be colder than absolute zero temperature. (The actual law is that nothing can reach exactly zero. The laser strives toward zero from below.) The laser doesn’t feel cold because it has a lot of heat, and when that heat is transferred to your body, it increases your entropy in the normal way: positive temperature. This is because the atoms in your body, like most materials, are not limited to two energy states.
The laser is an unusual example, but it shows us what a weird concept temperature can be, and how it has as much to do with information as it does with heat.
Since temperature is such an abstract concept, I’d like to try to convince weathermen to report the daily warmth in a more natural way— heat transfer rate through skin. As a unit of energy per time, this quantity would be measured in watts, and a chart would look a bit like this:

| −500 watts | naked in outer space (all heat leaves the body) |
| −200 watts | too damn cold! |
| −100 watts | cozy (normal human heat output) |
| 0 watts | like bathing in warm blood (literally) |
| anything more | too damn hot! |
It would be a very human-centric scale, since factors like body temperature and the way that humidity interacts with skin would have to be included in the calculation (or experiment, using a NIST-standard human). Leprechauns would have to get their own scale.

All artwork on this page was originally created by Arthur Rackham.